Ketogenic Diet in Cancer Prevention and Therapy
In recent years, there has been a renewed interest in understanding how altered metabolism contributes to cancer pathogenesis. Many factors, such as tumor hypoxia, stromal composition, immune cell infiltration, and genetic alterations, play critical roles in defining cancer cell metabolism. Genetic and/or epigenetic alterations can provide a crucial survival advantage to cancer cells in a nutrient-starved environment. The renewed interest in cancer cell metabolism came initially from recognizing that dysregulated signaling pathways and transcriptional reprograming result in metabolic alterations in cancer cells.
It has been shown that the induction of oncogenes and/or loss of tumor suppressors is/are sufficient to drive metabolic changes in cancer cells.Indeed, tumor-associated metabolic alterations are recognized as an emerging cancer hallmark.
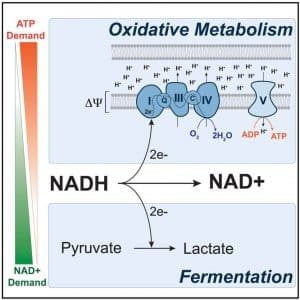
Energy is maximally released when glucose is completely oxidized to CO2, a process that produces ATP through oxidative phosphorylation (OXPHOS). When oxygen is limiting in cells, glycolysis becomes uncoupled from OXPHOS, and the end product of glycolysis, pyruvate, is instead reduced to lactate. Interestingly, in many cancer cells, glucose is preferentially catabolized through fermentation to lactate even when oxygen is not limiting (the Warburg effect). The conversion of pyruvate to lactate appears to be an essential mechanism by which cancer cells maintain an appropriate balance of redox cofactors to support biosynthetic functions. It has been shown that loss of TP53 activity can push cells toward the glycolytic pathway instead of OXPHOS.
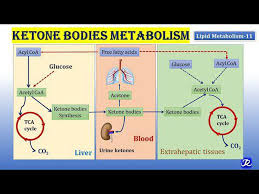
Cancer cells need to maintain a proper ratio of saturated to unsaturated fatty acids to avoid mitochondrial dysfunction, excess reactive oxygen species, and endoplasmic reticulum stress. In addition to impacting carcinogenesis, diet and lifestyle factors may also affect cancer therapy. The Ketogenic Diet (KD), a high-fat/low-carbohydrate/adequate-protein diet, has recently been proposed as an adjuvant therapy in cancer treatment. KDs target the Warburg effect, a biochemical phenomenon in which cancer cells predominantly utilize glycolysis instead of oxidative phosphorylation to produce ATP.
To date, evidence from randomized controlled clinical trials is lacking, but needed, to answer the question of whether an adjuvant KD would benefit specific cancer patients. Human data pertaining to KDs and cancer are mostly based on single case reports and a smattering of preliminary clinical studies with small study cohorts, heterogenous study designs, poor compliance to the diet, noncomparable regimens, or without standardized dietary guidance.
